What is RedETSA?
The Health Technology Assessment Network of the Americas (RedETSA) is a network formed of health ministries and institutions, health technology assessment agencies, regulatory authorities, collaborating centers of the World Health Organization (WHO) and the Pan American Health Organization (PAHO) and education and research institutions in the region of the Americas.
Our Network conducts regional meetings and distance learning programs for members and non-members. It includes the participation of 21 member countries in the Americas Region, represented by 41 health institutions that conduct Health Technology Assessments or are in the process of developing them.
This extensive collaborative network enables the exchange of valuable information to promote the Health Technology Assessment (HTA) process in the Americas, informing and supporting decision-making regarding the incorporation, use, and replacement of health technologies in health systems.
Our goals
Our objectives are aimed at improving the quality of health care, patient safety and the rational use of technologies. This contributes to the sustainability of health systems and equity in access.

VISIBILITY AND INSTITUTIONALIZATION: Identify the status of Health Technology Assessment (HTA) at the national and regional levels, as well as promote HTA processes to inform and support decision-making regarding the incorporation, use, and replacement of health technologies in the region's health systems.
ACCESS, DISSEMINATION AND INTEGRATION: Facilitate access to information and knowledge sharing on HTA through the Network and the Regional Database of Health Technology Assessment Reports in the Americas (BRISA)

ETHICS: Promote good practices for HTA.
TRAINING: Strengthen the skills of human resources in HTA in health systems.

SYNERGY: Facilitate cooperation between countries and institutions through networking and encourage the consolidation of existing local ETS networks and their synergy with RedETSA.
INFORMED DECISIONS: Reduce information asymmetry, contributing to improved evidence-based health decision-making processes.

COOPERATION: Promote cooperation with other HTA networks (national, sub-regional and global).
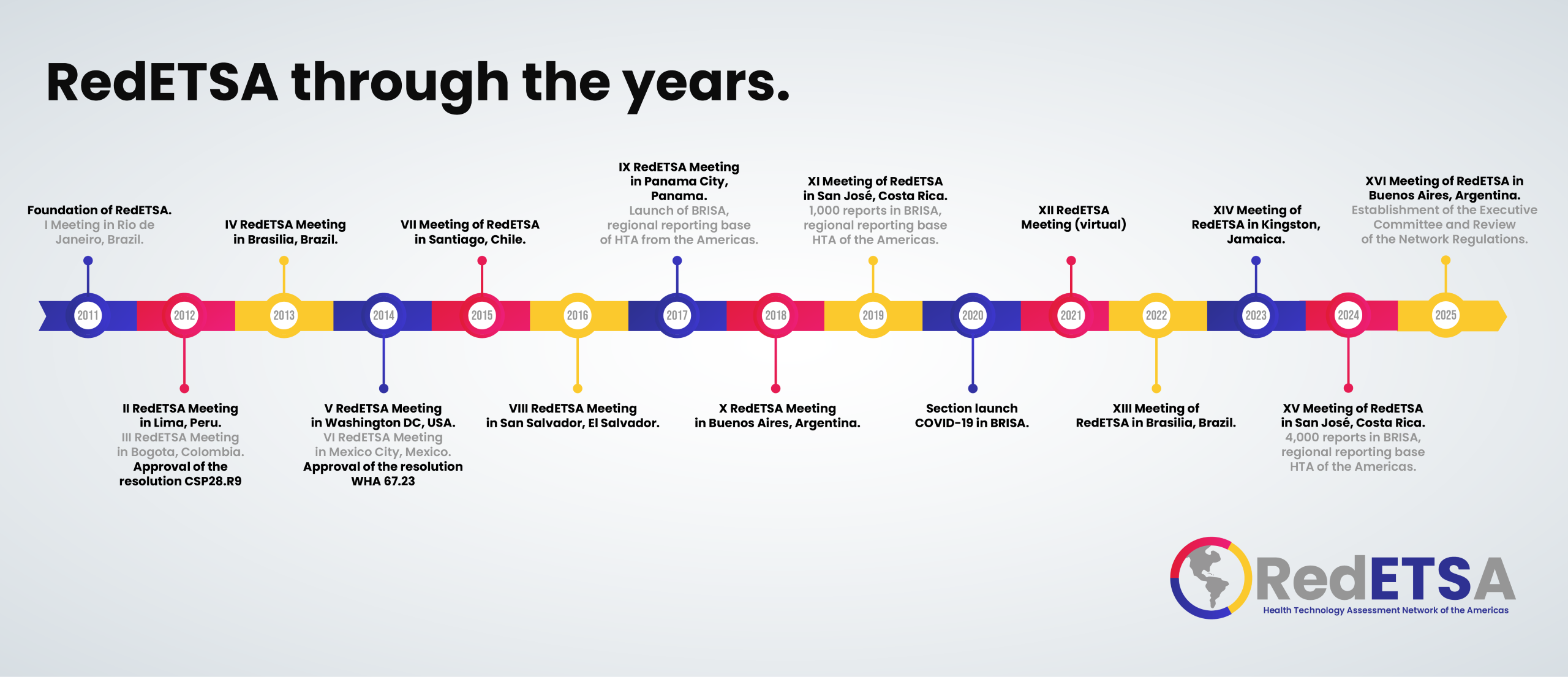
RedETSA History
One of the great challenges of health systems based on Primary Health Care is the search for equity, quality of care and efficiency. Health technologies play an essential role in this search. Not only are they decisive for the quality of care, but they also represent an increasing budgetary impact that can threaten the sustainability of health systems.
In this context, the decision on the technologies that should be provided by health systems is key for countries to obtain the maximum possible health benefits. It is noteworthy that regional cooperation in this matter has gained strength in recent years.
2010RedETSA is born
During the Regional Meeting on Health Technology Assessment held in Buenos Aires, in October 2010, representatives from 12 countries and a total of 20 institutions, including the countries' ministries of health, Collaborating Centers of the Pan American Health Organization ( PAHO) and the World Health Organization (WHO), and other networks and institutions, agreed to the creation of the Health Technology Assessment Network of the Americas (RedETSA).
2011Launch
This Network was officially launched in June 2011, in Rio de Janeiro, with the signing of cooperation agreements between the National Health Surveillance Agency (ANVISA) of Brazil, the Canadian Agency for Drugs and Health Technologies (CADTH) and the the United States for International Development (USAID). The objective was clear: to exchange information, promote the adoption of common methodologies and establish joint work priorities to strengthen capacities in Health Technology Assessment (HTA).
2012Workplan
A meeting was held in April 2012 in Lima, where the Network's priorities were defined, a work plan was established, and it was agreed that PAHO would exercise its secretariat. In September 2012, at the 28th Pan American Sanitary Conference, Member States were pioneers, worldwide, by adopting for the first time a resolution on the evaluation and incorporation of health technologies in health systems. Resolution CSP28.R9 was an innovative policy document that proposed linking evidence-based decision-making for health technology assessment with the decision-making process in health systems to manage and use technologies. sanitary. The importance of RedETSA was recognized in the resolution and Member States were urged to take part.
2021-202210 years of RedETSA
In 2021 RedETSA celebrated 10 years since its foundation. Due to the COVID-19 pandemic, that year the first and only virtual regional meeting of the Network was held.
In 2022, at the 30th Pan American Sanitary Conference, the final report of Resolution CSP28.R9 was presented, showing the progress made by countries in the last 10 years in the institutionalization and development of HTA as a fundamental tool to inform decision-making. health coverage decisions.
2023Members
Currently RedETSA is made up of 42 member institutions that represent 21 countries in the Region of the Americas.
PAHO/WHO Resolutions
CSP30/INF/12
PROGRESS REPORT
Access and rational use of medicines and other strategic and high-cost health technologies. (September 2022) Read more »
CSP30/INF/11
FINAL REPORT
Evaluation and incorporation of health technologies in health systems. (September 2022) Read more »
CD54/INF/5/F
PROGRESS REPORT
Evaluation and incorporation of health technologies in health systems. (September 2015) Read more »
WHA67.23
RESOLUTION
Assessment of health interventions and technologies in support of universal health coverage
(May 2014) Read more »
CSP28/11
WORK DOCUMENT
Evaluation and incorporation of health technologies in health systems. (September 2012) Read more »
CSP28.R9
RESOLUTION
Evaluation and incorporation of health technologies in health systems. (September 2012)Read more »